
Parkinson's disease is the second most common neurodegenerative disease after Alzheimer's disease. In China, the number of patients has exceeded 5 million. As the disease progresses, patients may experience symptoms such as uncontrollable tremors, bradykinesia, insomnia and memory decline. The onset of this disease is closely related to the progressive loss of dopaminergic neurons in the substantia nigra of the brain and the abnormal accumulation of originally soluble α-synuclein.
Levodopa is the main drug for the treatment of Parkinson's disease. It can quickly supplement the dopamine deficient in the brain, improve the motor symptoms of patients, and has significant clinical effects. However, long-term use of levodopa is prone to cause motor complications such as symptom fluctuations and dyskinesia. Moreover, it is usually effective in the early stage, and about half of the patients will experience the "end-of-dose phenomenon" (fluctuation of drug efficacy) after approximately five years.
In the stem cell therapy for Parkinson's disease, dopaminergic neural precursor cells obtained by stem cell differentiation are implanted into the patient's brain. These precursor cells will mature into functional dopamine neurons in the body, make up for the loss of neurons in the nigrostriatal pathway of Parkinson's disease patients, and improve motor dysfunction from the root cause. At present, stem cell therapy for Parkinson's disease has shown promising results.

In August 2025, Sumitomo Pharma and Racthera jointly announced the submission of a manufacturing and marketing authorization application for allogeneic induced pluripotent stem cell (iPSC)-derived dopaminergic neural progenitor cells (generic name: raguneprocel). This application is based on a Phase Ⅰ/Ⅱ open-label investigator-initiated trial (IIT) led by Kyoto University Hospital, and the relevant research results were published in the international top journal Nature in April this year.
In this trial, 7 patients with moderate-to-severe Parkinson's disease (aged 50–69 years) received unilateral or bilateral putaminal transplantation of raguneprocel and were followed up for 24 months after surgery. The results showed good safety and positive efficacy: the transplanted cells could survive long-term and produce dopamine, and patients' motor symptoms were improved, with the high-dose group showing particularly significant effects. Specifically, the average improvement in the "off" phase MDS-UPDRS Ⅲ score was 9.5 points (20.4%) among 4 patients, and the average improvement in the "on" phase score was 4.3 points (35.7%) among 5 patients.

In October 2025, Bayer Group and its wholly-owned subsidiary BlueRock Therapeutics announced that the 36-month data from the Phase I clinical trial of their stem cell therapy bemdaneprocel for Parkinson's disease continued to show favorable safety and long-lasting efficacy stability.
In October 2025, Bayer Group and its wholly-owned subsidiary BlueRock Therapeutics announced that the 36-month data from the Phase I clinical trial of their stem cell therapy bemdaneprocel for Parkinson's disease continued to show favorable safety and long-lasting efficacy stability.
Previously, bemdaneprocel had entered Phase III clinical research and completed the administration to the first patient.
Different from Sumitomo Pharma's raguneprocel, bemdaneprocel is a stem cell therapeutic agent composed of dopaminergic neural precursors differentiated from human embryonic stem cells. In addition, other types of stem cells have also been developed for the treatment of Parkinson's disease.
Various types of stem cells demonstrate differentiated potential in treatment
Stem cells that can be used for differentiation come from diverse sources, and the following types have attracted much attention due to their unique advantages:
Embryonic Stem Cells (ESCs): They can differentiate into target precursor cells in vitro and be transplanted into the brain to replace the lost dopaminergic neurons in patients. Due to their totipotency, ESCs can generate various cell types, thus having enormous therapeutic potential.
Induced Pluripotent Stem Cells (iPSCs): These cells are produced by converting somatic cells (such as skin cells, blood cells, etc.) into pluripotent cells with characteristics similar to embryonic stem cells through specific gene reprogramming technology. iPSCs can be derived from the patient's own body, which reduces immune rejection and avoids ethical issues.
Neural Stem Cells (NSCs): Neural stem cells can directly differentiate into neurons, astrocytes, etc. They can home to damaged brain regions, enhance connections between neural synapses, establish new neural circuits, and achieve regeneration of damaged neural tissues by secreting paracrine neuroprotective substances and neurotrophic factors.
Mesenchymal Stem Cells (MSCs): Mesenchymal stem cells have multiple differentiation potentials and the potential to replace lost dopaminergic neurons. They also have immunomodulatory and anti-inflammatory effects, which can protect nerves and resist apoptosis, as well as promote neurogenesis, angiogenesis, and improve cellular metabolism.
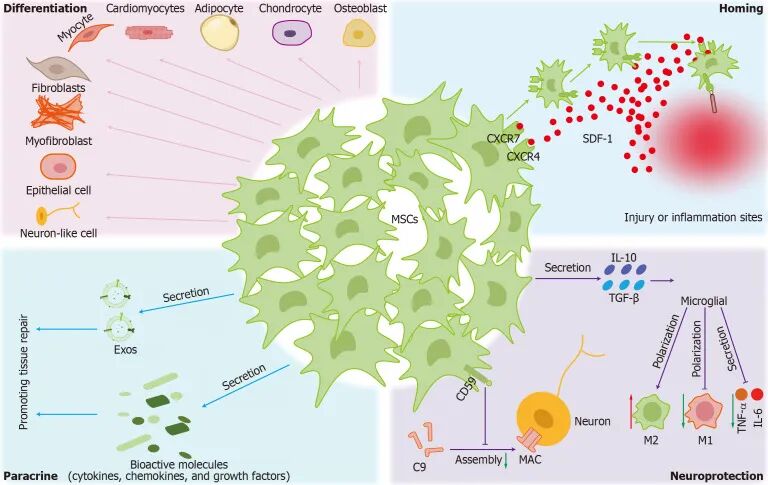
The Therapeutic Mechanisms of Mesenchymal Stem Cells
Multiple Pathways and Advances in Stem Cell Therapy for Parkinson's Disease
In addition to Sumitomo Pharma and Bayer, many other companies are actively laying out plans in the field of stem cell therapy for Parkinson's disease. Different technical pathways have collectively demonstrated the potential of cell therapy.
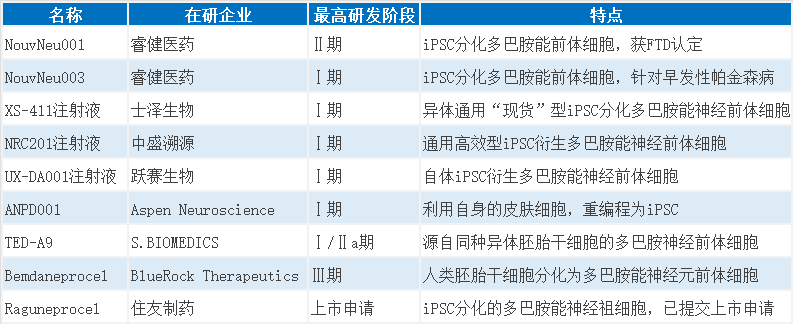
Progress in Some Investigational Stem Cell Therapies for Parkinson's Disease
Several IIT have also yielded promising results, expanding the approaches of cell replacement therapy in treating Parkinson's disease:
Capital Medical University conducted the first transplantation trial using dopaminergic precursor cells differentiated from autologous induced neural stem cells (NSCs). After surgery, patients experienced prolonged "on" periods, and "off" phase symptoms significantly improved compared to pre-operation;
Peking Union Medical College Hospital made the first attempt to transplant NSCs via the nasal mucosa route, with a single treatment leading to sustained symptom improvement for 12 months;
The University of Houston evaluated the potential of allogeneic bone marrow-derived MSC in treating Parkinson's disease. Following cell transplantation, motor function in the treatment group showed significant improvement.
In addition, gene therapy has demonstrated potential in treating Parkinson's disease. It alleviates symptoms by regulating neurotransmitters and controls disease progression by delaying neurodegeneration through neuroprotective genes. Gene therapy products such as RGL-193 by Ruidi Therapeutics, VGN-R09b by Tizeta Therapeutics, and BBM-P002 by Belief BioMed have all entered clinical research stages.
Kylin Lab facilitates the R&D of drugs for Parkinson's disease
Kylin Lab specializes in providing preclinical CRO services for central nervous system (CNS) drug development. In the research of Parkinson's disease drugs, it has established stable and professional animal models, such as mature models including MPTP-induced PD models and 6-OHDA-induced PD models. It also owns a comprehensive behavioral evaluation platform and a reliable biomarker detection platform. With extensive experience in preclinical research on Parkinson's disease therapeutic drugs as well as cell and gene therapy drugs, KylinLab can accurately evaluate the efficacy of candidate drugs from multiple dimensions, effectively improving the success rate of clinical translation of drugs.

References:
Sameer A Chaudhary, Sapana Chaudhary, Sakshi Rawat. Understanding Parkinson's disease: current trends and its multifaceted complications. Front Aging Neurosci. 2025 Sep 18:17:1617106.
Jong Mi Park, Masoud Rahmati, Sang Chul Lee, et al. Effects of mesenchymal stem cell on dopaminergic neurons, motor and memory functions in animal models of Parkinson's disease: a systematic review and meta-analysis. Neural Regen Res. 2024 Jul 1;19(7):1584-1592.
Weizhao Chen, Qiaoying Huang, Shanshan M, et al. Progress in Dopaminergic Cell Replacement and Regenerative Strategies for Parkinson's Disease. ACS Chem Neurosci. 2019 Feb 20;10(2):839-851.
Zhiguo Chen, Guoguang Zhao. First-in-human transplantation of autologous induced neural stem cell-derived dopaminergic precursors to treat Parkinson’s disease. Sci Bull (Beijing). 2023 Nov 30;68(22):2700-2703.
Nobukatsu Sawamoto, Daisuke Doi, Etsuro Nakanishi, et al. Phase I/II trial of iPS-cell-derived dopaminergic cells for Parkinson’s disease. Nature. 2025 Apr 16;641(8064):971–977.
Kylin Lab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.