
Recently, the neuroscience sector witnessed another major financing event: MapLight Therapeutics announced the successful closure of a $372.5 million oversubscribed Series D round. This funding not only set a new annual record for financing in the central nervous system (CNS) field but also ranked as the third-largest biopharmaceutical financing deal globally in 2025. This follows MapLight's previous $225 million funding round in 2023, marking another significant capital endorsement in the CNS arena.

This financing round was co-led by Forbion and Goldman Sachs Life Sciences Fund, with participation from new investors including Sanofi and T. Rowe Price, alongside continued support from existing shareholders such as Novo Holdings. The funds will be used to advance the company's leading drug candidate through Phase II trials for schizophrenia and Alzheimer's disease-related psychosis, as well as to explore other potential indications for ML-007C-MA.。
The new capital will also support the development of other assets in MapLight's pipeline: a candidate for autism spectrum disorder is currently in Phase II trials, while programs targeting Parkinson's disease and hyperactivity/impulsivity disorder are in the preclinical research stage.
ML-007C-MA: A Novel Therapy Targeting Muscarinic Receptors
ML-007C-MA is a combination muscarinic drug designed for treating neurological and neuropsychiatric disorders. Its core mechanism involves a dual approach of 设"precision delivery + side effect blockade"”: by pairing agonists targeting M1 and M4 muscarinic acetylcholine receptor subtypes with precisely matched muscarinic antagonists, it delivers M1/M4 agonist activity specifically to the brain for therapeutic effects while effectively blocking peripheral side effects.
Muscarinic acetylcholine receptors (mAChRs) are widely distributed neurotransmitter receptors in the brain, primarily classified into five subtypes: M1 to M5.
Deficiencies in M1 receptors are linked to schizophrenia, and their direct modulation plays a critical role in psychiatric and cognitive neural circuits. M4 receptor modulation is important in complementary neural circuits related to psychosis. M1 receptors are highly expressed in the hippocampus and prefrontal cortex, directly regulating neural circuits associated with psychotic symptoms and cognitive function. Their activation may also hold potential therapeutic value for depression and generalized anxiety disorder. M4 receptors regulate complementary neural circuits related to psychosis and are considered potential targets for treating reward circuit disorders such as alcohol use disorder and gambling addiction.
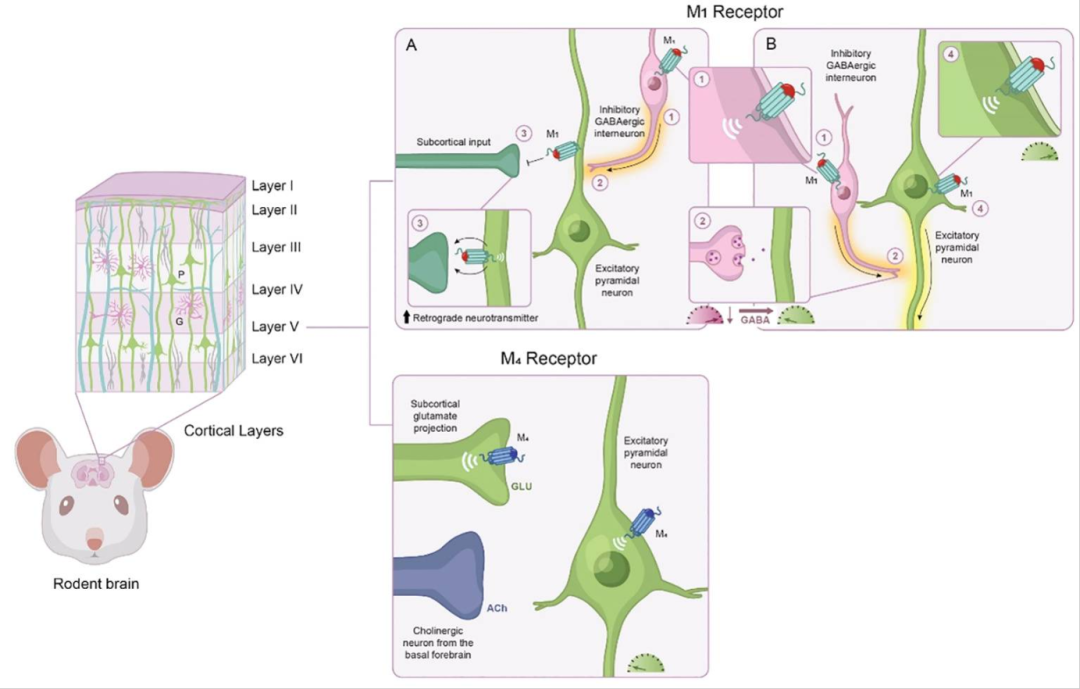
Regulatory Role of M1 and M4 mAChR Activation in the Frontal Cortex
This is not the first time mAChR-targeted therapies have attracted such significant attention. The success of the approved drug KarXT (an M1-M4 agonist) has already validated the clinical and commercial potential of this class of drugs.
In 2021, Zai Lab partnered with Karuna, securing exclusive rights for the development, manufacturing, and commercialization of KarXT in Greater China. In December 2023, Bristol Myers Squibb (BMS) announced its acquisition of Karuna for $14 billion, highlighting the strategic value of this therapy. In September 2024, KarXT received FDA approval for treating schizophrenia in adults, generating $10 million in sales in its first full quarter (Q4 2024). In early 2025, China's National Medical Products Administration (NMPA) accepted the New Drug Application (NDA) for KarXT, accelerating its launch in China.
Global Neuroscience Investment Enters a High-Growth Cycle
Rise of China's CNS Drug R&D Ecosystem
As global aging intensifies and the burden of neurological diseases grows, companies with breakthrough technology platforms are attracting substantial investment. In 2025, financing activity in the global neuroscience sector continues to surge, with multiple innovative companies securing capital and several nine-figure funding deals emerging.
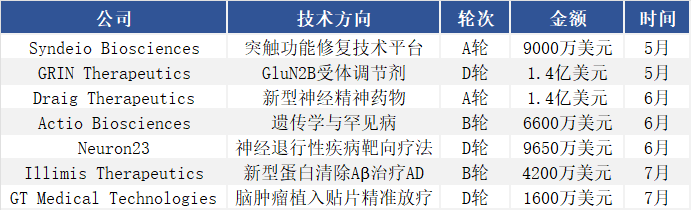
May-July 2025, Financing Data of Select Global CNS-Related Companies,
China has also demonstrated strong growth. In the first half of 2025, at least 24 innovative drug companies, including Glinton Pharmaceutical, Darui Biotech, and NeuShen Medicine, completed financing rounds totaling over RMB 11 billion, forming a multi-tiered innovation echelon. In the second half of the year, CNS financing remains active, with companies like Darwin Bioscience receiving further capital support.
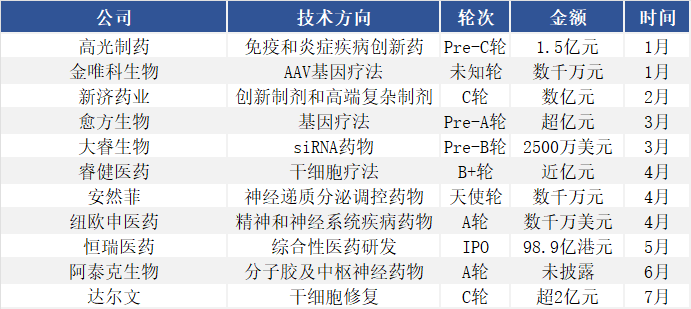
January-July 2025, Financing Data of Select Domestic CNS-Related Companies
A series of financing developments confirm that China's CNS drug R&D ecosystem is rapidly emerging and gaining significant importance within the global CNS drug development landscape.

References:
Chris J van Koppen, Björn Kaiser. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther. 2003 May;98(2):197-220. doi: 10.1016/s0163-7258(03)00032-9.
Samantha E Yohn, Phillip D Harvey, Stephen K Brannan, The potential of muscarinic M1 and M4 receptor activators for the treatment of cognitive impairment associated with schizophrenia. Front Psychiatry. 2024 Oct 4:15:1421554. doi: 10.3389/fpsyt.2024.1421554.
Brian Dean. IUPHAR Review on muscarinic M1 and M4 receptors as drug treatment targets relevant to the molecular pathology of schizophrenia. Pharmacol Res. 2024 Dec:210:107510. doi: 10.1016/j.phrs.2024.107510.
KylinLab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.