

KylinLab Contributes to the Clinical Translation of "NP001 Injection"
KylinLab is honored to provide preclinical research services for NOVABIOTX's Treg cell product, supporting its clinical translation. As a preclinical CRO company deeply specialized in central nervous system (CNS) disorders, we remain committed to advancing effective clinical translation through our professional preclinical research capabilities!

On September 23, 2025, the Investigational New Drug (IND) application for Human Autologous Polyclonal Regulatory T Cell Injection (referred to as "NP001 Injection"), independently developed by Shanghai NOVABIOTX Co., Ltd. (abbreviated as "NOVABIOTX") for treating Multiple System Atrophy (MSA), was accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). Acceptance Number: CXSL2500817.
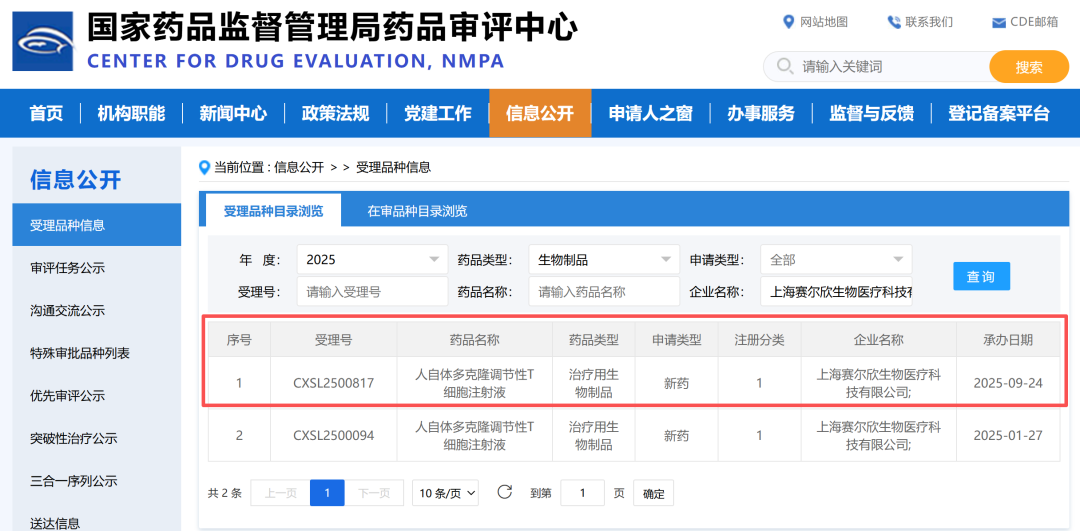
Previously, this candidate product had obtained clinical trial implied permission for Amyotrophic Lateral Sclerosis (ALS, commonly known as Lou Gehrig's disease). The acceptance of this IND for the MSA indication marks another critical step forward in its clinical exploration within the field of neurodegenerative diseases.
Dr. Lü Mingqi, CEO of NOVABIOTX, stated: MSA is characterized by relatively difficult early diagnosis and poor prognosis. Currently, global treatments for MSA primarily focus on symptomatic support, with no specific therapies available to delay or halt disease progression. The acceptance of this IND application for the MSA indication is not only a significant milestone for NOVABIOTX in the field of neurodegenerative diseases but also indicates that Treg cell therapy has the potential to extend to a broader range of neurodegenerative disorders. We hope this product can provide a new treatment option and bring new hope to MSA patients who respond poorly to existing medications.
About Multiple System Atrophy
Multiple System Atrophy (MSA) is an adult-onset, progressive, and fatal neurodegenerative disease with an unclear etiology. It is typically sporadic and may be associated with genetic factors (such as mutations in COQ2, SNCA genes) and limited environmental exposures. Its clinical features mainly include various combinations of autonomic dysfunction, parkinsonism, cerebellar ataxia, and pyramidal tract signs, making early diagnosis difficult and prognosis poor. MSA can be classified into the MSA-P type, dominated by parkinsonism, and the MSA-C type, dominated by cerebellar syndrome, with MSA-P being more common. Pathologically, it is characterized by α-synuclein-positive glial cytoplasmic inclusions in oligodendrocytes, accompanied by multisystem neural structure atrophy or degeneration. Epidemiology shows that the prevalence of MSA is approximately 1.9 to 4.9 cases per 100,000 people, increasing in populations aged 40 or 50 and above; the average age of onset is around 56-60 years, with a median survival period of about 8-9 years after onset.
About Treg Cells
Regulatory T cells (Tregs) have become a therapeutic focus for autoimmune diseases and neuroinflammatory-related conditions due to their key roles in immune tolerance, inflammation suppression, and neuroprotection. Their core advantage lies in blocking the vicious cycle of neuroinflammation through multiple mechanisms, such as inhibiting overactivated microglia, regulating cytokine secretion, and repairing the blood-brain barrier, offering a novel approach to treating neurodegenerative diseases.
About Human Autologous Polyclonal Treg Injection (NP001)
"A Phase I/IIa Clinical Study on the Safety and Efficacy of Human Autologous Polyclonal Regulatory T Cells (NP001 Cell Injection) in Patients with Amyotrophic Lateral Sclerosis" is currently being conducted at the Neurology Center of Beijing Tiantan Hospital, Capital Medical University.
“"An Open-label, Single-arm, Single-center Clinical Study Evaluating the Safety and Tolerability of Regulatory T Cell Therapy for Neurodegenerative Diseases" is advancing at the First Affiliated Hospital of Zhengzhou University. To date, 8 patients have been enrolled. Data from patients who have already received reinfusion show highly encouraging results. Multiple efficacy indicators demonstrate positive clinical improvements, suggesting the potential for effective control of disease progression in the future.
About NOVABIOTX
Shanghai NOVABIOTX Co., Ltd. commenced operations in early 2023, focusing on developing multimodal Treg cell therapies for neurodegenerative diseases, autoimmune diseases, and metabolic diseases. Years of exploratory research have revealed that Tregs can effectively suppress the proliferation and function of pro-inflammatory cells in autoimmune diseases through multiple pathways, thereby achieving therapeutic effects. Upholding the core mission of "Addressing Unmet Clinical Needs" and the core values of "Patient-centricity, Integrity, Pragmatism, and Perseverance," NOVABIOTX is leading the industrial development of Treg therapies.

Sources:
[1]Notice from the General Office of the National Health Commission on Issuing the Guidelines for the Diagnosis and Treatment of Rare Diseases (2019 Edition)
[2]"Chinese Expert Consensus on Diagnostic Criteria for Multiple System Atrophy (2022)" Chinese Journal of Neurology, January 2023, Vol. 56, No. 1
KylinLab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.