
Voltage-gated Sodium Channels (Nav) play a pivotal role in regulating membrane excitability by generating and propagating action potentials to control electrical signals. Dysfunctions or dysregulations of Nav channels are associated with a variety of pathological conditions, such as epilepsy, cardiac arrhythmias, myopathies and pain.
The Nav channel family is widely distributed in organisms, with each subtype exerting specific functions in different tissues and cells. Among them, Nav1.7, Nav1.8 and Nav1.9 are predominantly expressed in the peripheral nervous system (PNS), such as nociceptive dorsal root ganglion (DRG) neurons, and participate in the perception and transmission of pain signals. Thus, Nav1.7-1.9 have become important targets for the development of potent non-addictive analgesics.

Distribution of Voltage-gated Sodium Channel Subtypes and Associated Diseases
Nav1.7 Analgesic Drug Development
Nav1.7 is encoded by the SCN9A gene and is primarily expressed in small-diameter peripheral sensory neurons, sympathetic ganglion neurons, trigeminal ganglion sensory neurons and vagal sensory neurons. By responding to membrane depolarization, Nav1.7 mediates the generation and repetitive firing of action potentials, playing a central role in electrical signal conduction in nociceptive sensory neurons. Genetic evidence also indicates that gain-of-function or loss-of-function mutations of Nav1.7 are associated with enhanced or diminished pain sensation, respectively. Genetic evidence also indicates that gain-of-function or loss-of-function mutations of Nav1.7 are associated with enhanced or diminished pain sensation, respectively.
尽管目前多款高选择性阻断剂在临床中未能展现令人满意的镇痛疗效,但Nav1.7仍是镇痛药物开发的优质靶点,各大制药公司对Nav1.7阻滞剂的投资热情不减。利用多样化的分子策略以及更加精准的体外抑制活性评价方法和疼痛动物模型, 并关注药物代谢动力学特征,Although several highly selective Nav1.7 blockers have failed to demonstrate satisfactory analgesic efficacy in clinical trials to date, Nav1.7 remains a promising target for analgesic development, and pharmaceutical companies continue to invest enthusiastically in Nav1.7 blocker research. With the adoption of diverse molecular strategies, more precise in vitro inhibitory activity assays and pain animal models, along with a focus on pharmacokinetic characteristics, the development of Nav1.7-targeted analgesics still holds great potential.
Tetrodotoxin, developed by WEX Pharmaceuticals—a wholly owned subsidiary of Cheung Kong Life Sciences—has been granted Fast Track Designation by the U.S. Food and Drug Administration (FDA) for the treatment of chemotherapy-induced neuropathic pain. Multiple drug candidates, including ANP-390 developed by Alphanavi and DWP-17061 developed by In Therapeutics, have entered clinical trial stages.
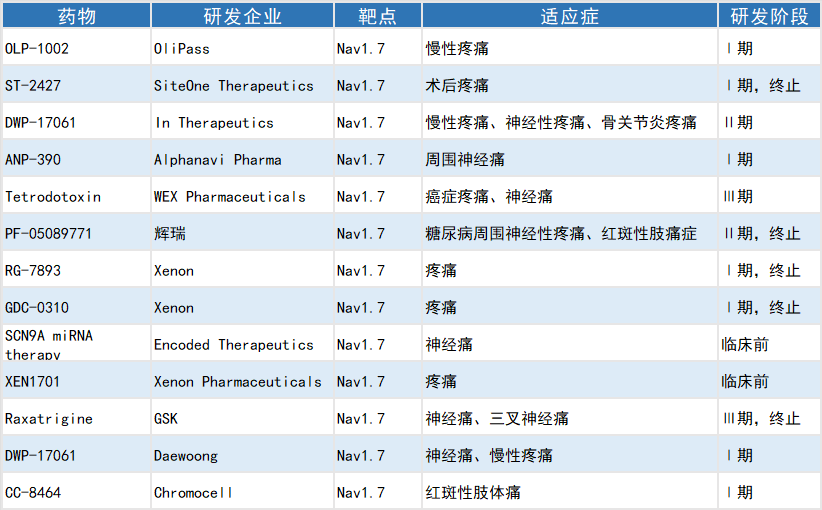
Selected Global Investigational Nav1.7 Blocker Analgesics
Nav1.8 Analgesic Drug Development
NNav1.8 is primarily involved in inflammatory and neuropathic pain in the peripheral nervous system. In depolarized DRG neurons, it exhibits rapid recovery from inactivation and enables high-frequency repetitive firing. This property provides a mechanistic explanation for Nav1.8’s critical role in neuronal hyperexcitability and pain signal transmission. Coupled with its restricted peripheral distribution, Nav1.8 has emerged as a key therapeutic target for the development of novel analgesics.
Since 2005, drug development targeting Nav1.8 has advanced rapidly, with numerous pharmaceutical companies laying out related pipelines. However, some drug candidates have been discontinued due to issues such as poor bioavailability and insufficient clinical efficacy. Nevertheless, these R&D challenges have not hindered innovative breakthroughs in this field. In January 2025, Vertex Pharmaceuticals’ Suzetrigine—endowed with high selectivity for Nav1.8 and supported by positive Phase Ⅲ clinical data in postoperative acute pain—was approved by the FDA, becoming the first non-opioid drug for acute pain treatment in over two decades. Meanwhile, companies including Latigo, GSK, MSD and Eli Lilly are actively advancing the development of Nav1.8-targeted drugs.
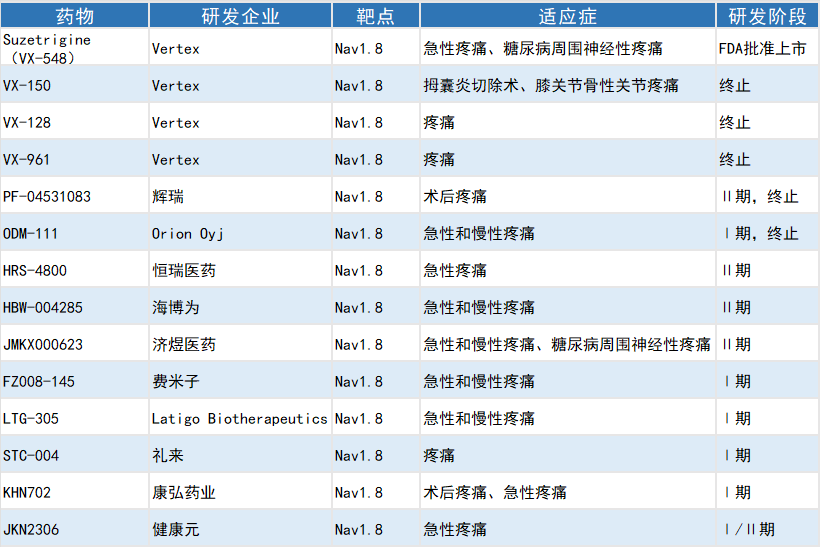
Selected Global Investigational Nav1.8 Blocker Analgesics
Domestic pharmaceutical enterprises such as Shanghai Huilun Pharmaceutical, Humanwell Healthcare, Insilico Medicine, Sichuan Kelun Pharmaceutical, Nanjing Qingpu Biotech, Wuhan Xirui Pharmaceutical, Shanghai Wennai, Sunshine Angenz, NeuroMend Therapeutics and Jiangsu Jichuan Pharmaceutical have also filed patents for Nav1.8-related compounds.
Multi-channel Synergistic Blockade Strategy
Alongside the active research on Nav1.7 and Nav1.8 subtypes, some companies are exploring the simultaneous inhibition of multiple pain pathways to achieve analgesic effects.
ANP-230, developed by Alphanavi, exerts dose-dependent analgesic effects by blocking Nav1.7, Nav1.8 and Nav1.9. Its efficacy is enhanced with repeated administration, and it exhibits favorable safety profiles. The drug has entered Phase Ⅱ clinical trials in Japan.
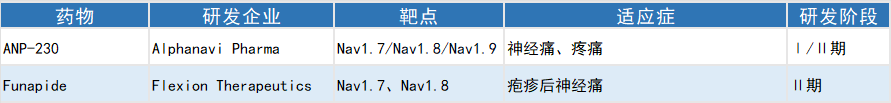
Selected Global Investigational Multi-target Sodium Channel Blocker Analgesics
As a breakthrough direction in pain management, Nav1.7 and Nav1.8 blockers possess the dual core advantages of potent analgesic efficacy and non-addiction. We anticipate that these blockers will transform traditional pain management paradigms and spearhead a new revolution in the global analgesic market.
Kylin Lab Supports Pain-related Drug Development
Kylin Lab boasts a seasoned electrophysiology team and robust technology platforms, equipped with comprehensive capabilities in patch-clamp recording, whole-cell potential monitoring, voltage clamping and ion channel current measurement. These enable precise detection of compound-mediated modulation of various ion channels. Meanwhile, relying on its efficient high-throughput screening platform, the company can rapidly and large-scale screen compound libraries, systematically evaluate the biological activity and mechanism of action of compounds at the ion channel and receptor levels, and provide reliable data support for early drug discovery and safety assessment.

References:
KylinLab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.