
October CNS Drug Development Trends and Progress
JEBEL's Class 1 Antidepressant New Drug Shows Superior Safety in Phase Ⅲ Trial
On October 30, JEBEL announced the completion of the Phase Ⅲ clinical trial for its Class 1 antidepressant new drug JJH201501. Study results indicate that a 10mg dose of JJH201501 achieves therapeutic effects equivalent to 20mg of vortioxetine, with a lower incidence of adverse reactions. The incidence of adverse events leading to treatment discontinuation is even comparable to that of the placebo group.
As the first Class 1 new drug developed on JEBEL's deuteration technology platform, JJH201501 is derived from vortioxetine. The deuteration platform replaces hydrogen atoms at specific sites in drug molecules with deuterium atoms to improve the pharmacokinetics or reduce the toxicity of the drug. Optimized through deuteration technology, JJH201501 demonstrates better key safety indicators and has the potential to become a first-line clinical drug.
Lundbeck's Migraine Drug Eptinezumab Submitted for First Domestic Marketing Approval
On October 22, Lundbeck submitted a marketing application for eptinezumab to the NMPA. This monoclonal antibody has been launched in more than 30 markets worldwide, providing an effective treatment option for migraine patients. Eptinezumab is a humanized monoclonal antibody that specifically targets calcitonin gene-related peptide (CGRP) and is administered intravenously.
A study primarily involving Asian patients showed that during Weeks 1-12, the 300mg dose group of eptinezumab achieved a 7.5-day reduction in monthly migraine days (MMDs), the 100mg dose group a 7.2-day reduction, while the placebo group only saw a 4.8-day reduction. These results are consistent with previous studies in multi-ethnic populations. Additionally, eptinezumab exhibits rapid-onset preventive efficacy.
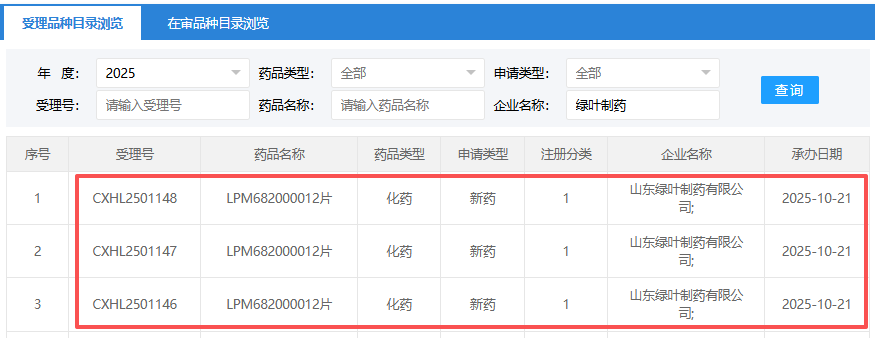
Luye Pharma's Antidepressant New Drug Receives IND Acceptance
In the field of depression, Luye Pharma continues to explore. On October 21st, the official website of the China National Center for Drug Evaluation (CDE) announced that the clinical trial application for Class 1 small molecule drug LPM682000012 tablets submitted by Luye Pharma has been accepted and is intended for the treatment of depression. Its specific mechanism of action has not been disclosed yet. The main purpose of this experiment is to evaluate the safety and tolerability of a single dose of this drug in healthy subjects; The secondary objective is to evaluate the pharmacokinetic (PK) characteristics and food effects of its single administration. The first clinical approval of LPM682000012 tablets in China was granted in November 2024.
4B Tech's "First-in-Class" Monoclonal Antibody Enters U.S. Clinical Trials
On October 9, 4B Tech announced that its independently developed first-in-class NGF receptor allosteric inhibitor, 4B03-04 Injection, recently received Investigational New Drug (IND) clearance from the U.S. FDA. A Phase Ⅰ study targeting osteoarthritis pain will be initiated in the United States. Adopting a novel mechanism of action, the drug delivers effective analgesia while avoiding the side effects of traditional NGF-targeted drugs and has no addictive potential. Early studies show excellent safety and a long half-life, enabling the potential for once-monthly administration. It brings breakthrough hope for addressing the significant clinical need in moderate-to-severe chronic pain treatment.
Bayer's Cell Therapy for Parkinson's Disease Releases Positive Phase Ⅰ Data
On October 6, BlueRock Therapeutics, a subsidiary of Bayer, announced positive 36-month Phase I clinical trial data for its investigational cell therapy bemdaneprocel in the treatment of Parkinson's disease. The results demonstrate that the therapy consistently maintains good safety and tolerability. Even after discontinuing immunosuppressive treatment, the transplanted cells continue to survive in the brain.
We look forward to continued breakthroughs in new drug development in the CNS field, bringing more and better treatment options to patients.

Source:
Mario García-Domínguez. 2025: 1-22.NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities. Curr Issues Mol Biol. 2025 Jan 31;47(2):93.
https://www.cde.org.cn/main/xxgk/listpage/9f9c74c73e0f8f56a8bfbc646055026d
https://mp.weixin.qq.com/s/kg_luxWBLzgkluu83lN2eQ
https://mp.weixin.qq.com/s/yo1yF1_D182HHNed2Ws6Sg
Kylin Lab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.