
The 2025 Nobel Prize in Physiology or Medicine has been awarded to three scientists who have made groundbreaking contributions in the field of "peripheral immune tolerance" - Mary E. Brunko, Fred Lamsdale, and Shigefumi Sakaguchi. Their research revealed the core role of regulatory T cells (Tregs) in suppressing autoimmune responses.
As a subset of CD4 ⁺ T cells with critical inhibitory functions, Treg cells play a core role in "braking" traditional immune responses, maintaining immune balance, and preventing excessive immunity. Their main mechanisms include regulating antigen-presenting cells and inhibiting the activation of effector T cells; Release anti-inflammatory cytokines and reduce the activity of immune cells; Using metabolic intervention to inhibit the proliferation and activation of peripheral T cells, or directly killing overactivated effector T cells.
However, under pathological conditions, Treg cells may exhibit abnormalities such as decreased quantity, weakened function, or defects. Supplementing Treg cells or enhancing their function has become a new direction for treating related diseases such as cancer, inflammation, and autoimmune diseases.
In addition to immune regulatory functions, studies have also revealed the important potential of Treg cells in promoting central nervous system repair and regeneration: Dombrowski et al.'s research shows that Treg cells can promote oligodendrocyte differentiation and myelin regeneration; Ito et al. found that the regulatory protein AREG produced by Tregs helps prevent the formation of inhibitory scar tissue, thereby promoting neuronal survival and axonal regeneration.
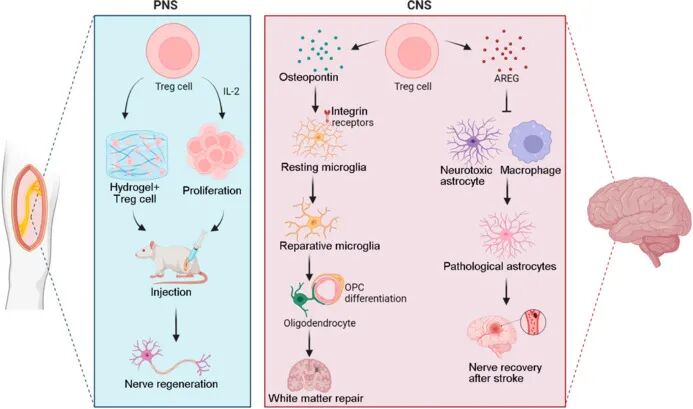
The role of Treg cells in neurological diseases and regeneration
The mechanism by which Treg cells maintain immune balance and promote damage repair may provide new solutions for autoimmune responses in diseases such as ALS.
Focusing on ALS immune imbalance, Treg cells bring new therapeutic possibilities
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease characterized by the gradual death of motor neurons, severely impairing the patient's ability to move their hands and feet, speak, swallow, and ultimately breathe.
At present, the etiology of ALS is not clear, and it is generally believed to be related to factors such as genes and environment. In recent years, research has shown that the onset of ALS is closely related to an imbalance in the autoimmune system
• The ability of Treg cells isolated from the blood of ALS patients to inhibit the proliferation of effector T cells is poor;
• In rapidly progressing ALS patients, the number of Treg cells and the expression of their marker FOXP3 are reduced, indicating the presence of functional Treg cell defects in the disease progression;
• The latest research shows that ALS patients have an autoimmune response against the C9orf72 protein on nerve cells; patients with higher levels of anti-inflammatory factor IL-10 release in the body are predicted to have significantly prolonged survival.
These findings collectively suggest that using Treg cells to treat ALS has the potential to all eviate the condition and prolong patient survival.
Treg cells can inhibit overactivated effector T cells and alleviate immune responses in patients; Simultaneously promoting the repair of nerves and their myelin tissue. Although this strategy does not directly target the root cause of ALS, it can prevent further nerve damage through immune regulation, there by delaying disease progression. 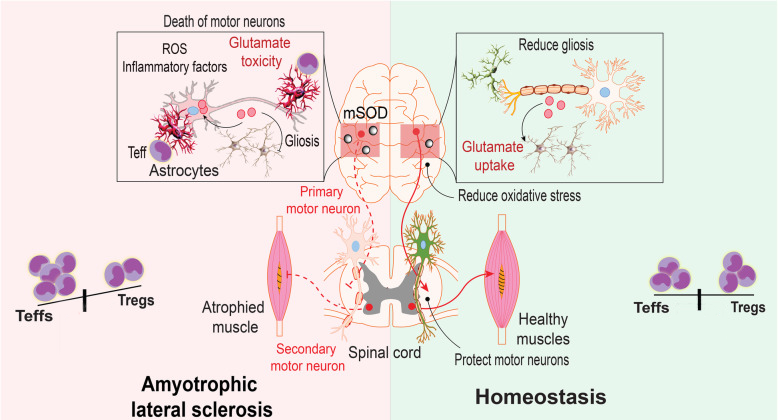
The immune imbalance role of Treg cells in the pathological process of ALS
Global Treg cell therapy accelerates ALS transformation
Nowadays, global research on Treg cell therapy has made breakthrough progress in the field of ALS, and multiple clinical studies have demonstrated the effectiveness of Treg cell therapy
• Cellenkos' "spot type" Treg product CK0803 has been recognized as an orphan drug by the US FDA. Currently, in phase Ⅰ clinical trials, preliminary data shows that after a single infusion, the average rate of functional decline in patients slows down by 60%;
• The world's first intrathecal injection Treg therapy developed by Shanghai Saierxin Biomedical Technology Co., has been approved by NMPA to enter phase Ⅰ/Ⅱa clinical trials. The first patient has recently completed drug administration, and this method is expected to allow cells to reach the lesion more directly;
• Coya Therapeutics announced the release of Phase Ⅱa clinical trial data for the treatment of ALS with COYA 101, with most patients experiencing slowing or cessation of progression during the 24 week open label extension period
Treg therapy, from theory to clinical practice, relies on solid preclinical research verification at every step. In this transformation chain, Hanshu Biotechnology, with its deep layout in the CNS field and multidimensional research platform, provides key support for the mechanism exploration and efficacy evaluation of innovative therapies such as Treg, helping to accelerate clinical translation of research.

References:
KylinLab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.