Dec 27, 2023, the clinical trial application for Nuoshen Pharma's NS-041 orally disintegrating tablets was accepted by China's NMPA.
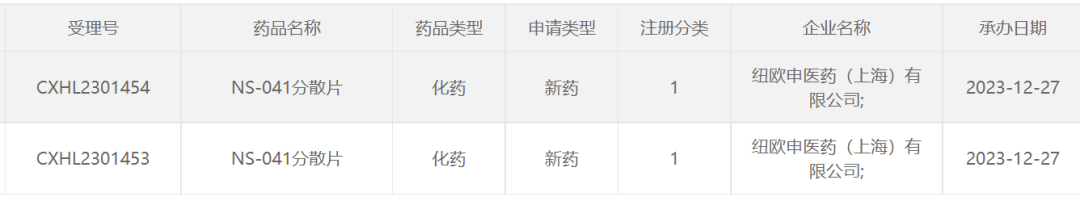
According to the official website of NeuShen, NS-041 is a KCNQ2/3 (Kv7.2/Kv7.3) activator intended for the treatment of indications such as epilepsy and major depressive disorder (MDD).
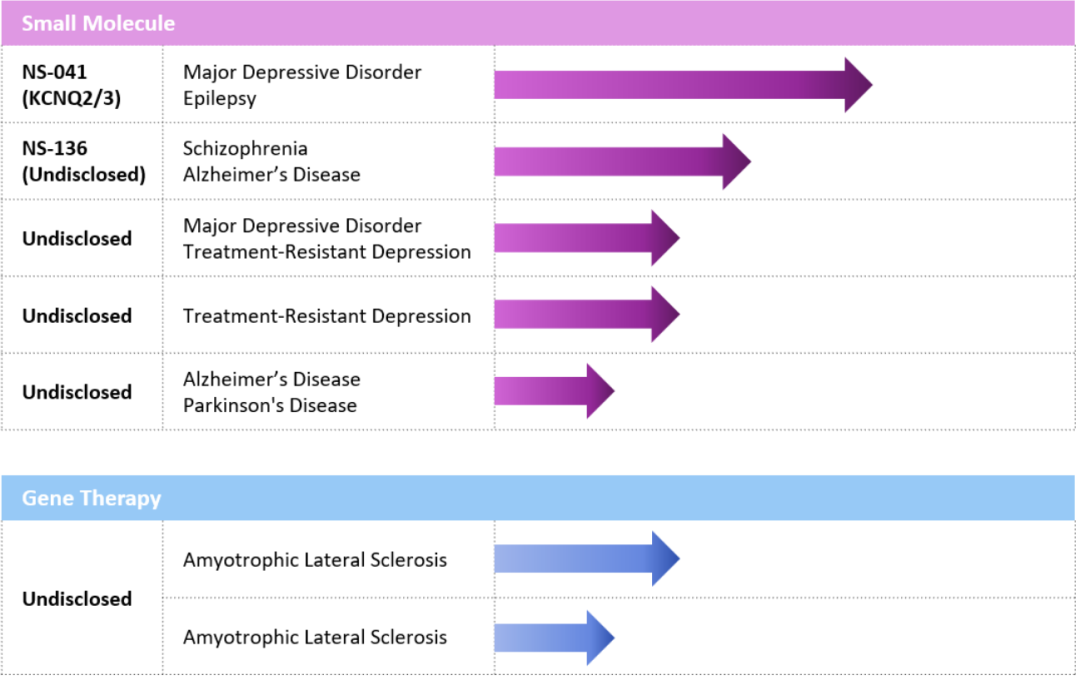
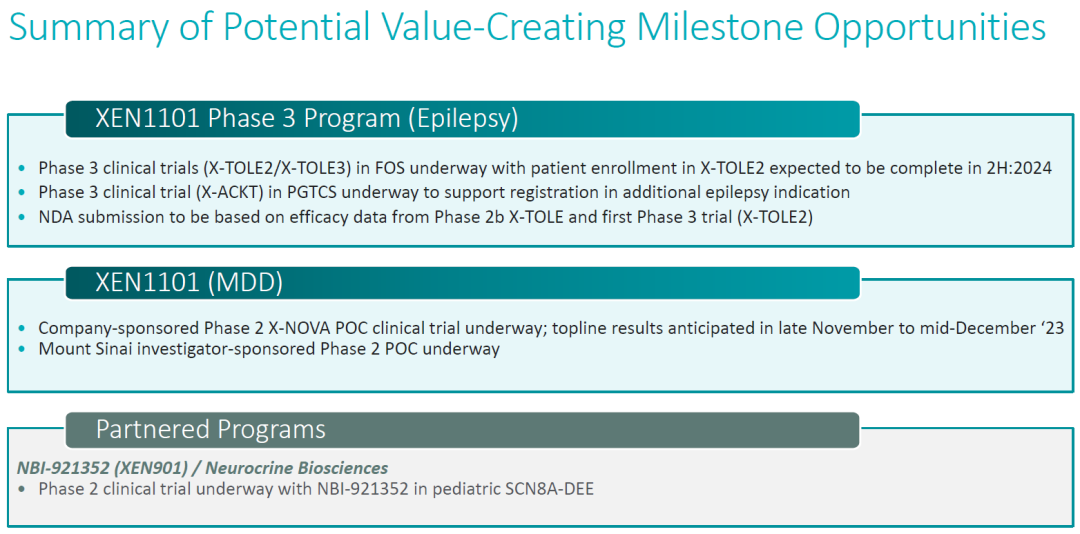
Globally, the clinical progress of Kv7 activators has been rapid, with Xenon's XEN1101 having advanced to Phase III clinical trials.
XEN1101在2b期临床中表现出优异疗效。
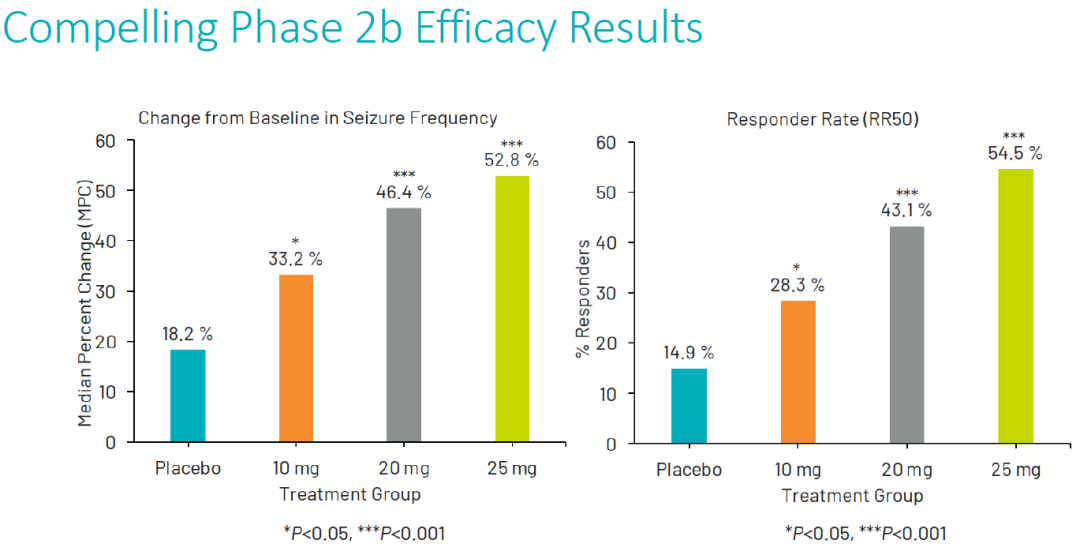
XEN1101 demonstrated exceptional efficacy in its Phase IIb clinical trial.
Summary
The past two years have witnessed significant clinical breakthroughs in the development of novel drugs for CNS disorders, an area receiving growing attention within the industry. Particularly in China, where the high difficulty of R&D had long resulted in relatively few participants, an increasing number of innovative biotech companies have emerged in recent years.
Source: 医药笔记

Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.
(扫码关注)👇
