
Menopausal Vasomotor Symptoms (VMS), also known as hot flashes, are characterized by hot flushes and night sweats. They are one of the most common manifestations in perimenopausal and postmenopausal women, which may last for more than a decade, and also a major reason why women seek medical treatment for menopausal symptoms.
The mechanism of VMS is complex. Current studies suggest that its core mechanism is as follows: during perimenopause, the decline in estrogen levels leads to abnormal activation of the NKB-NK3R signaling axis in the arcuate nucleus neurons of the hypothalamus, narrowing the thermoregulatory window and triggering VMS episodes.
NK3 Receptor Antagonists
Based on the above mechanism, NK3R has become a key therapeutic target for hot flashes. NK3R antagonists can block the NKB-NK3R signaling pathway, ameliorate hot flashes and stabilize body temperature.
At present, Fezolinetant, the first NK3R antagonist, was approved for marketing by the FDA in May 2023, and approved for introduction in the Guangdong-Hong Kong-Macao Greater Bay Area in October 2025. According to Astellas' financial report, the sales volume of Fezolinetant reached $65,078,400 (RMB 467 million) in the first quarter of fiscal year 2025, a year-on-year increase of 45.6%.
NK1/NK3 Dual Receptor Antagonists
The success of NK3R antagonists has triggered further research and attention on the tachykinin family. Among them, NK1, the receptor for substance P, is involved in physiological processes such as mood regulation, anxiety, pain modulation and vasodilation, and also has the potential to be developed as a drug for the treatment of menopausal symptoms.
Tachykinins and Their Receptors
Elinzanetant developed by Bayer is an NK1/NK3 dual receptor antagonist that targets NK1 receptors. While blocking the NKB-NK3R signaling pathway, this drug also inhibits the Substance P-NK1R pathway. Its phase III clinical trial results showed that Elinzanetant significantly reduced the frequency and severity of VMS, improved sleep disorders and menopause-related quality of life, and demonstrated good safety.
Currently, Elinzanetant has been approved for marketing in the UK, Canada, the United States and other regions. Clinical trials in China are underway, and it is expected to submit a new drug application between 2026 and 2027.
Clinical Insights into the R&D of New Drugs for Hot Flashes
What are the unmet clinical pain points in the treatment of hot flashes? Can NK3R antagonists and NK1/NK3 dual receptor antagonists break the treatment dilemma? In the process of new drug R&D, what core preclinical data do clinicians focus on? How should clinical trials be scientifically designed? With these questions, we interviewed clinical experts in this field. Let's listen to their professional answers.
Q
From the patients you have treated, what are the most urgent problems to be solved in the current treatment of hot flashes?
A
First, hormone replacement therapy (HRT) has safety risks and restrictions on contraindicated populations, and most patients are unwilling to receive hormone therapy. Second, emotional anxiety and sleep disorders that coexist with hot flashes are also urgent problems to be solved. Therefore, the overall symptoms of menopause require comprehensive treatment.
Q
Based on your clinical diagnosis and treatment experience, what is the scale of patients with hot flash-related symptoms and their acceptance of new drugs?
A
There are about 180 million perimenopausal women over 45 years old in China, among whom the incidence of moderate to severe hot flashes is 30%—50%. Patients have many concerns about hormone therapy, but have a high acceptance of non-hormonal new drugs (such as NK3R antagonists). These drugs are more likely to be popularized if they have low side effects, especially low hepatotoxicity.
If new drugs can improve sleep, anxiety and other problems while treating hot flashes, they will further enhance patient acceptance and market penetration rate.
Q
What are the limitations of current single-target NK3 drugs (fezolinetant) in clinical use?
A
Its improvement rate for hot flashes is about 50%-60%. In particular, it does not completely reduce night sweats, resulting in limited improvement in sleep quality.
In addition, it has no significant effect on mood disorders.
Q
Is it possible for NK1+NK3 dual-target drugs to provide more comprehensive symptom improvement than single NK3-target drugs?
A
From the perspective of mechanism of action, NK3 is involved in body temperature regulation, while NK1 is involved in the regulation of mood, vasodilation and pain. The synergy of NK1 and NK3 dual targets may provide more comprehensive symptom improvement than single NK3-target drugs, especially with more significant effects in relieving night sweats, anxiety and sleep disorders.
Therefore, clinicians generally believe that dual targets are more likely to "comprehensively improve menopausal syndrome".
Q
Do doctors think there is sufficient evidence that NK1 is involved in the regulation of hot flashes?
A
The evidence that NK3 is involved in the regulation of hot flashes is very clear, but the role of NK1 is more indirect. It mainly triggers or aggravates hot flashes through mood, anxiety and vasodilation, rather than directly regulating the thermoregulatory center.
Q
Regarding NK1+NK3 dual-target drugs, what preclinical data are expected to be seen?
A
The expected preclinical data include: body temperature telemetry data; the effect of NK1 inhibition on hot flush frequency; whether the improvement of anxiety can reduce the threshold for hot flash triggering, etc.(For more detailed information, please scan the QR code below, add Kylin Lab's WeChat official account, and reply "hot flashes")

Q
What do you think are the most important study endpoints that should be included in clinical trials of relevant drugs?
A
Hot flash frequency; nocturnal hot flashes; sleep scores; anxiety scores (GAD-7); liver function changes; quality of life indicators (MENQOL); etc.
Kylin Lab Animal Model of Hot Flashes
Kylin Lab adopts the senktide-induced hot flash model, which directly targets the hypothalamic NKB/NK3R signaling pathway that is known to be overactive in human menopausal hot flashes, with a clear mechanism and high physiological and pathological relevance.
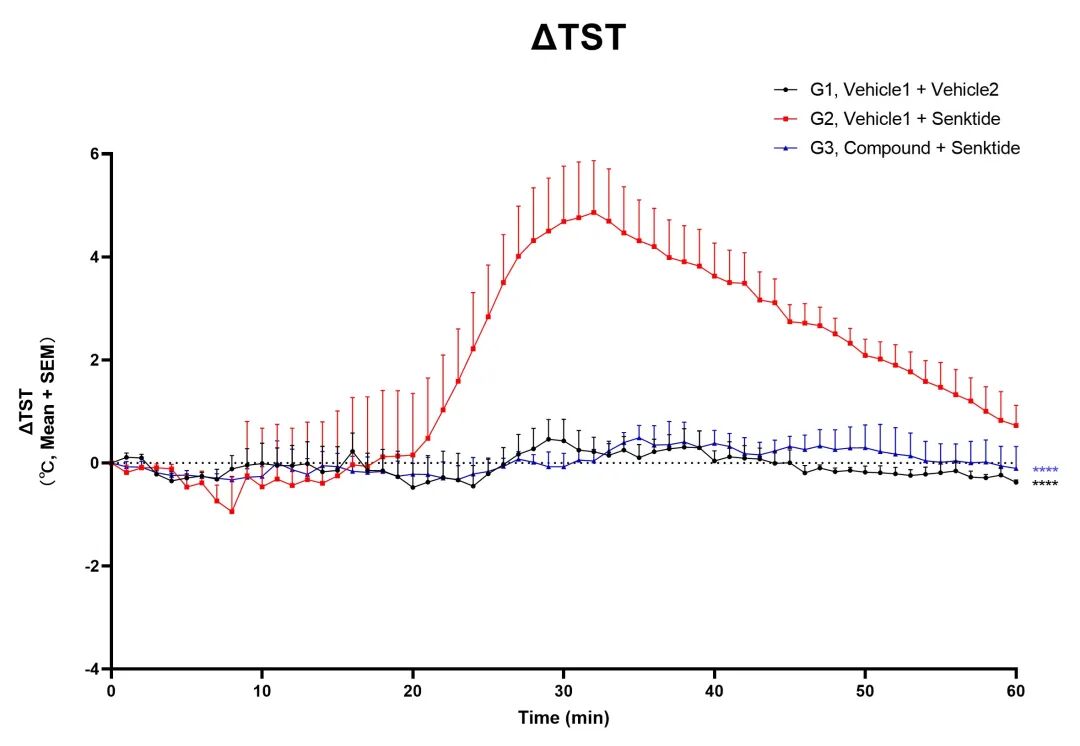
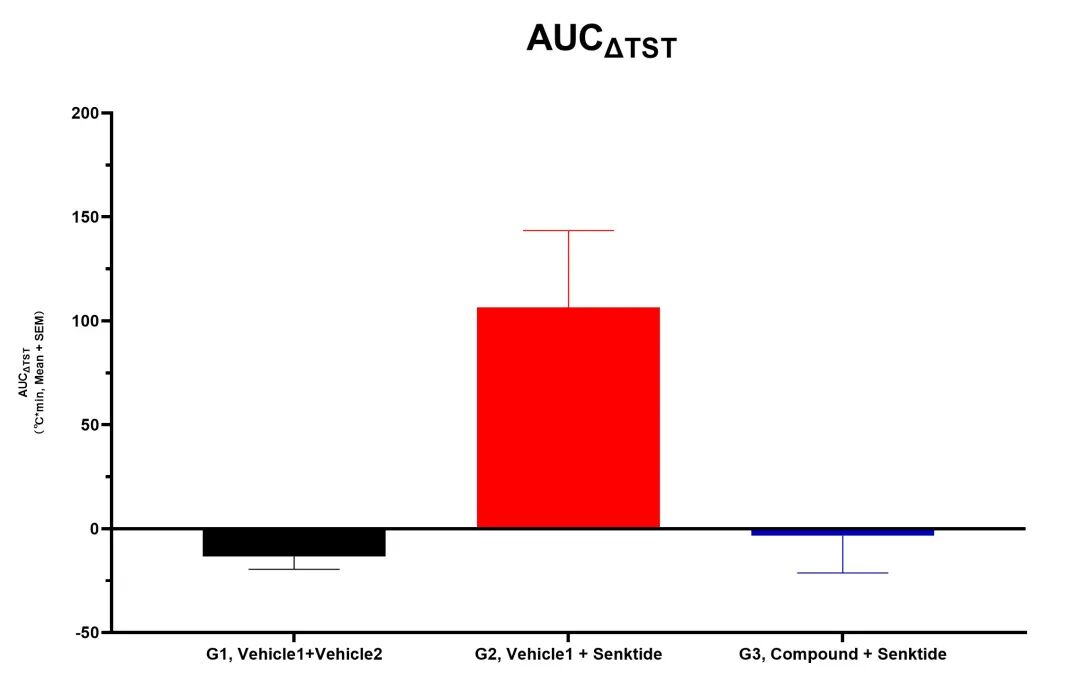
In animals treated with effective compounds, hot flash symptoms were significantly improved.

Disclaimer:
This article aims to popularize medical and health information and share industry trends. All contents are only for knowledge dissemination and academic discussion, and shall in no way constitute any form of medical advice, diagnosis and treatment plan, or medication guidance. Readers must consult qualified professional physicians for any decisions related to their own health.
The discussions in this article regarding the scale of patients with menopausal symptoms, analysis of treatment needs and drug market potential are all based on publicly available industry data and expert opinions, which are only descriptions of the current situation and discussions on trends. They do not constitute a judgment on the commercial value of any drug or company, nor are they investment advice.
Readers are requested to use the information in this article based on rational and prudent judgment. The author and the publishing platform shall not be liable for any direct or indirect losses incurred by any person relying on the contents of this article or taking actions accordingly.
KylinLab
CNSxplore | Pioneering CNS Drug Discovery--In vitro and in vivo, Beyond limits
Kylin Lab is a preclinical CRO company specializing in central nervous system (CNS) diseases, dedicated to offering one stop solutions for CNS drug discovery. With a portfolio of fully-validated cellular and animal disease models, combined with comprehensive research and analytical capabilities, we empower clients to accelerate the development of innovative therapies and reduce clinical trial risks.
Kylin Lab boasts an experienced technical team well-versed in international regulations, along with high-standard experimental platforms. Our core technologies include AI-driven phenotypic screening, humanized stem cells and organoids, electrophysiology, high-throughput electroencephalography (EEG), histology, and molecular biology. Our expertise spans a broad range of areas including Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, spinal muscular atrophy (SMA), pain, and stroke etc.
Since its establishment, Kylin Lab has adhered to the philosophy of “Pioneering R&D, Leading in technology Quality-first, Customer-centric.” We have served hundreds of clients, successfully completed numerous thematic research projects and IND submissions, and established a high-quality, stable, and forward-looking efficacy evaluation system.